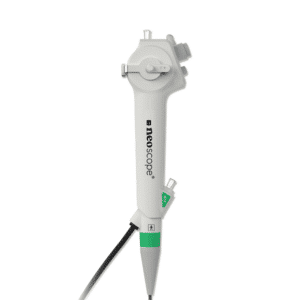
Water-soluble and non-irritating, contains no fat or alcohol. Compatible with mucous membranes, latex-free, phthalate-free, and Bisphenol A-free.
REFERENCES & PRODUCT INFORMATION
Manufacturer, class and certification listed under the reference table - Single-use medical devices for general care - Please refer to the package insert for further information. Check the integrity of the packaging before use.
Asept InMed - N° identification 383600590, RCS Toulouse B 383 600 590 - The products presented are medical devices - The manufacturer, the class and the certification of the DM are informed in the legal mentions of the brochure - Non contractual photos - Information intended for health professionals.
Our team
Our specialists - hospital representatives - are there to accompany you in the discovery and testing of our products.

Alexis Marie
Product Specialist

Cyrielle Verdoncq
Product Specialist
Jordan Hugonin
Sales coordinator - Ile de France

Freddy Cliquet
Hospital Delegate - Southwest
Romane Estevon
Hospital Delegate - East

Ermin Uzunovic
Hospital delegate - Paca

Mélanie Botros
Hospital delegate - Ile de France

Pierre-Emmanuel Paré
Hospital Delegate - North West

Alexandre Novelli
Hospital Delegate - Rhônes-Alpes
You are a health professional and you want more information? Contact them!
Follow our news